White Blood Cell Donors
Information for those interested in being a Donor
We are recruiting two kinds of volunteers for this research study: White Blood Cell donors and cancer patients. The success of this trial for this cancer treatment depends on the white blood cells of many donors. Your cells may contain the activity that saves the lives of cancer patients. The cancer patients participating in this trial have already tried conventional therapies, and they have not responded to it. We urge you to seriously consider volunteering for this research study by becoming part of the Donor Registry for this trial.
Who can donate?
What are the risks and benefits to me if I participate?
What will happen to a donor during the study?
Sign up for the Donor Registry
Donating blood or blood components is a noble act that saves over 4.5 million lives every year, and this study provides an extraordinary opportunity for volunteers to save the life of a cancer patient who has no other treatment options. If you are seriously interested in volunteering for this research study by being a part of our Donor Registry, please read the following information before you complete the online DONOR REPLY FORM.
Time commitment
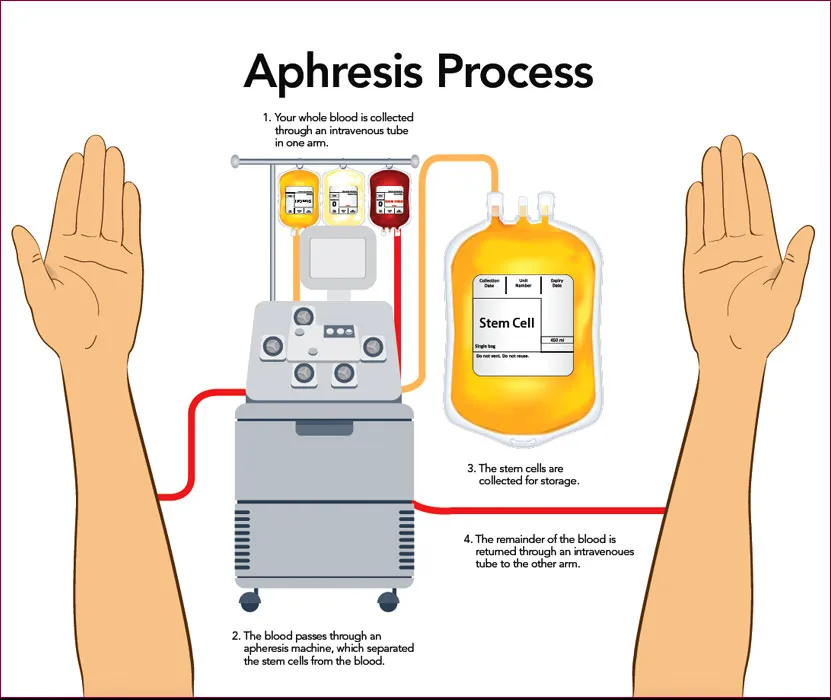
We estimate that at least four visits to the South Florida Bone Marrow/Stem Cell Transplant Institute (SFBMSCTI) in Boynton Beach, Florida will be needed for each qualified donor. At each of two separate initial visits we will collect a small amount of whole blood from you to perform a series of descriptive laboratory tests to determine if you are a candidate donor. Additionally, you will receive a physical exam from a physician. If the results show that you would be an appropriate donor, we will ask you to come to the SFBMSCTI when a suitable patient is identified. On this day, a physician will go over the procedure with you and make sure that you are well enough to donate the following day. You will then be given two medications (see below) to increase the number of white blood cells available for collection on the following day. On the day of collection, you return to the SFBMSCTI and undergo a two- to three-hour white blood cell donation called White Blood Cell apheresis, a process that is similar to plasma or platelet donation. Once collected and screened, your cells will be transfused for use in the cancer patient.
We anticipate that we’ll need 4 to 6 qualified donors per patient. Given our patient enrollment, this means we need several hundred eligible donors to go through the first two visits in the selection process. Individuals who are qualified will then be asked to complete the physician visit, take the medication, and then donate white blood cells. Eligible donors must be:
Able and willing to sign Donor Consent
Meet the Universal Healthy Eligible Blood Donor criteria (including no history of cardiac illness)
Donor must NOT be blood relatives to the patient/recipient
Able and willing to take dexamethasone and filgrastim
Able to undergo white blood cell collection by apheresis
Able and willing to undergo the standard and study-specific laboratory blood tests and physical exam in order to be considered a qualified donor.
A list of potential donors will be kept in a Donor Registry specific for the 08001-BMSCTI clinical trial after initial qualification, and individual donors will be notified when a donation is requested. You can withdraw from the Donor Registry at any time. All records will be stored according to HIPAA regulations.
If you meet the above requirements and would like to know more about donating your white blood cells for this clinical trial, please complete our online DONOR REPLY FORM. A research coordinator will contact you.
White Blood Cell donation is a safe procedure that has been used in the clinical setting for more than several decades. It is impossible to contract infectious diseases during White Blood Cell donation. The impact of donating White Blood Cells on a donor’s health is also minimal since White Blood Cells regenerate very quickly after donation. However, White Blood Cell donation is not completely without side effects. Please review the following information with your doctor and contact us regarding specific questions or concerns. Also please note that these details will be reviewed again with our clinical coordinator before you agree to participate.
Some donors may experience the potential side effects of filgrastim , dexamethasone , apheresis and hetastarch (also called Hespan).
During apheresis, although rare, some donors may experience chills, shakes, numbness and tingling of the hands and feet, lightheadedness or rarely fainting. These reactions are reversible by replacing calcium by pills or by injections. Some donors may experience a moderate decrease in the platelet count. More severe side effects in healthy donors are not completely impossible. The entire procedure may take approximately two and one half hours of your time. A registered nurse will be in constant attendance, and a physician will be readily available if needed.
Approximately 25% of donors receiving dexamethasone will experience restlessness, insomnia, and/or facial flushing for up to 24 hours.
Some mild to moderate pain primarily over the lower back or sternum may occur after administration of filgrastim and can be relieved with medications such as Tylenol. Filgrastim is widely used in a variety of settings and one-time use for White Blood Cell mobilization is considered highly safe.
Exposure to Hespan during apheresis is safe but may cause allergic reactions in rare occasions, such as shortness of breath and low blood pressure. Hespan may also cause a mild rash, which should dissipate with time. There are reports of a reversible short-lived hemophilia-like syndrome and inflammation of the pancreas with the use of Hespan.
Removal of White Blood Cells does not present a significant risk because White Blood Cells regenerate quickly. The FDA allows a healthy individual to donate White Blood Cells by apheresis up to 24 times a year, almost once every other week.
Please use the related links to find more information about the medications. You will be given complete information, in writing, about potential side effects during your first visit before you sign a consent form.
1.After reviewing the online information provided by this website, fill out the online DONOR REPLY FORM. need link to Donor Reply Form If you are selected to participate, our Clinical Coordinator will contact you to schedule your first visit.
2.At the first visit, you will review study information with the clinical coordinator where you will be able to ask study questions. If you agree, you will sign the consent form that details this information. In addition to consent, you will be given the Universal Blood Donor Questionnaire. A small amount of blood will be collected for initial screening tests.
3.Following the results of these tests you will be scheduled for a second visit at SFBMSCTI. During this second visit, you will meet with the study physician and undergo a physical and additional blood will be drawn for further testing to assure that your general health is suitable for donation. Other tests to determine your suitability as a donor may also be necessary (such as chest X-ray, EKG, etc.), similar to the tests performed when a person is cleared as a bone marrow transplant donor.
4.If you are a suitable donor for a specific patient you will be contacted for a final consult the day before your white blood collection. At this time you will be given two medications (dexamethasone and filgrastim).
5.The following day you will return to the SFBMSCTI facility. Prior to white blood cell collection you will again be administered the Universal Blood Donor Questionnaire. You will undergo 2 to 3 hours of white blood cell donation (White Blood Cell apheresis), which is similar to platelet donation.
Your name, contact address, and these results will be kept confidential in the study’s Donor Registry here at the South Florida Bone Marrow/Stem Cell Transplant Institute.
If you have read the information about who qualifies as a white blood cell donor for this research study, and you think you would like to donate white blood cells, please complete the Donor Reply Form.
Be sure you fill out the contact information completely, and click the SUBMIT button when you are done. If you are initially found to be a potential donor, a study coordinator will contact you to schedule your first visit.
Go to the Donor Reply Form
Interested in becoming a Donor?
For additional information regarding this trial, please call the South Florida Bone Marrow/Stem Cell Transplant Institute research team at 561-752-5522.
Request An Appointment
Please fill out the form to request an appointment. We will contact you shortly to confirm the exact day/time.