Cancer Patients
Additional Information and FAQ’s for Cancer Patients
The answer might be yes. Furthermore, if cancer is the consequence of a diminished activity of cancer resistance, we might be able to treat cancer by restoring this activity in cancer patients via transferring this activity from the donors who have high levels of it.
In 1999, a research team at Wake Forest University encountered a mouse that unexpectedly survived repeated injections of an especially lethal form of cancer that usually kills all laboratory mice. This cancer-fighting trait was inherited by the mouse’s offspring, and it was subsequently found that the cancer cells were killed by the mouse’s own white blood cells. The research team successfully demonstrated that the ability to kill cancer cells can be transferred from one mouse to another. It was also learned that a similar but more dynamic CKA exists in the white blood cells of humans. It is possible that this activity also represents a similar ability to eradicate cancer cells in human bodies.
This protocol has been developed based on the profound CKA in the granulocytes of some humans and the unprecedented results from preclinical efficacy studies in mice.
Given the surprising results of the mouse studies the researchers set out to find similar CKA in the white cells of healthy humans. Blood from a large number of people was tested and it was found that humans do indeed have CKA similar to the cancer resistant mice. In humans, however, CKA levels vary more between individuals; this variability may explain why some develop cancer and others do not.
In order to determine whether CKA levels are associated with cancer, evaluation of the CKA from people who were either healthy or had already been diagnosed with cancer was done. People with cancer had lower CKA levels than healthy people in a similar age group.
The types of leukocytes that displayed the highest level of CKA were the granulocytes and monocytes, which is very similar to what the researchers found in the cancer resistant mice. Interestingly, the CKA was lower in older subjects, during winter months or in times of stress.
This investigational study employs a treatment regimen that is significantly different from other cell therapies that have used either the patient’s own cells or a different type of donor cells, such as T-lymphocytes. This is the first time that donors will be selected based on criteria relevant to the population believed to have the ability to kill cancer cells.
Read more about:
How donors are selected
Who can participate?
What are the risks and benefits to a cancer patient who receives this granulocyte infusion therapy?
What will happen to patients during the study?
Once you have read about the study, complete the SUBJECT REPLY FORM to contact us.
You are capable of caring for yourself
You are up and about for at least half of the time each day
You don’t have diabetes, significant cardiac disease and/or an active serious infection
You have not used immunosuppressive agents other than steroids within 30 days of the trial
You are not pregnant or nursing
Your life expectancy must be at least 4 to 6 months
You have not received bone marrow / stem cell transplants
You have no evidence of brain metastases
You have not received treatment with fludarabine
You don’t have a hematologic malignancy
You can no longer benefit from conventional therapy
Common
Febrile nonhemolytic reaction – an elevated body temperature of 2oF during or shortly after a transfusion without hemolysis (evidence of destruction of red blood cells).
Allergic reactions – usually occur as urticaria (hives), but may also include wheezing, severe shortness of breath in rare cases.
Hemolytic transfusion reaction – the destruction of transfused red cells.
Immune-mediated platelet destruction that causes a lower platelet count and, in rare cases, can cause post-transfusion purpura (PTP) with sudden drop of platelet counts and bleeding, typically 7-10 days after a blood transfusion.
Uncommon/Rare
Transfusion-related acute lung injury (TRALI) occurs when a lot of fluids and protein leaks into the lungs, usually within 6 hours of transfusion, probably due to the presence of granulocyte antibodies in the donor or recipient.
Transfusion-associated Graft-versus-host disease (TAGVHD ) is a rare but extremely dangerous condition that occurs when viable T-lymphocytes in the transfused component engraft in the recipient and react against tissue antigens in the recipient. GVHD can occur if the host does not recognize the transfused cells as foreign and rejects them. This reaction can follow transfusion of any component that contains even very small numbers of viable T lymphocytes. Severely immunocompromised recipients are at greatest risk (e.g., fetuses receiving intrauterine transfusions, recipients of transplanted marrow or peripheral blood progenitor cells, and selected patients with severe immunodeficiency conditions), but GVHD has been reported in immunologically normal recipients
Cytomegalovirus (CMV) may, unpredictably, be present in white-cell-containing components from donors previously infected with this virus, which can persist life-long despite the presence of serum antibodies. Up to 70% of donors may be anti-CMV positive. Transmission of CMV by transfusion may be of concern in persons with decreased immunity, if they are CMV seronegative. For at-risk recipients, the risk of CMV transmission by cellular components can be reduced by transfusing CMV seronegative or leukocyte-reduced components. Other infectious agents include Babesia spp., Bartonella spp., Borrelia spp., Brucella spp., the agent of Colorado tick fever, Leishmania spp., Parvovirus spp., plasmodia, rickettsia, Toxoplasma spp., West Nile virus, and certain trypanosomes.
Bacterial contamination
Fluid overload (excessive fluid), hypothermia (decrease body temperature), hypocalcemia (decreased calcium level in the blood) and changes to potassium levels are rare unless large amount of transfusion is given.
Description
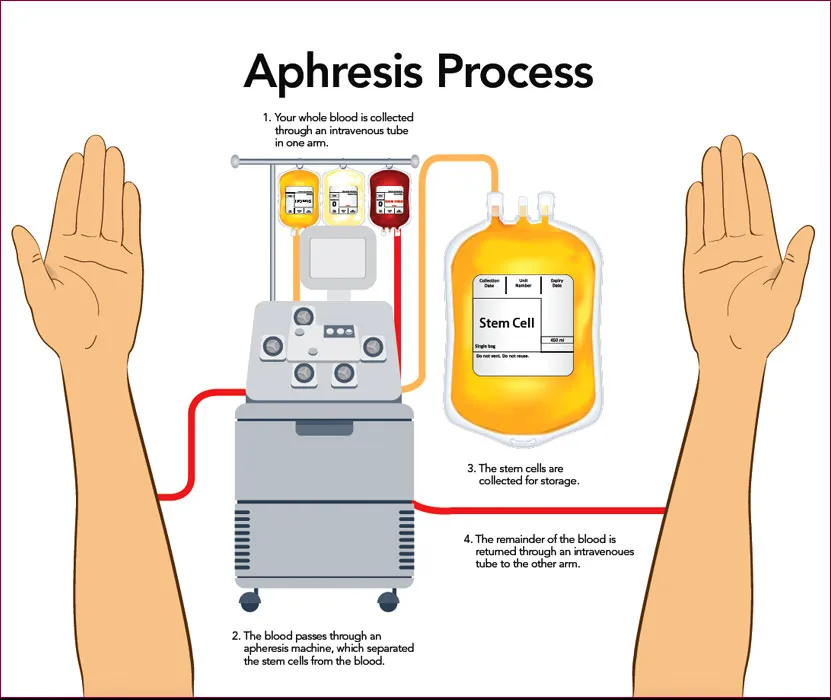
Granulocyte concentrates are typically collected in a process called leukapheresis. Granulocytes infusion usually contains many other leukocytes and platelets as well as 20-50 ml of red cells. The number of granulocytes in each concentrate is 1.0 × 1010, with the final volume of 200-400 mL including anticoagulant and plasma. Red cell sedimentation agents approved by the FDA, such as hydroxyethyl starch (HES), are typically used in the collection of granulocytes. Granulocytes infusion should be administered as soon after collection as possible due to deterioration of granulocyte function on storage. Infusion should occur no more than 24 hours after collection.
Side effects of granulocyte infusion include those described above. The following side effects are more common to granulocyte infusion:
Febrile Nonhemolytic Reactions; chills, fever, and shortness of breath may occur in patients receiving granulocyte components
Allergic Reactions: Allergic reactions to HES and other red cell sedimenting solutions may occur during granulocyte transfusion.
Although white cell transfer may provide benefits to you in the form of regression of your cancer/disease and increased long-term survival, it may also be associated with a significant risk of complications and even death resulting from the treatment. The actual risk of serious complications and death is difficult to estimate accurately – it depends upon age, physical condition, number of prior therapies received, type of donor, etc.
Risks of biopsy – There is a potential risk of bleeding, infection or nerve damage with a biopsy procedure. A biopsy is normally performed before treatment, however, a repeat biopsy of your tumor after therapy, is not standard of care and may be done for research study purposes.
Reproductive Risks – This treatment has an unknown risk of causing malformations in an unborn child, especially when given in the early part of pregnancy. Therefore, you should not become pregnant or father a baby while on this study. For this reason, both men and women will be asked to practice an effective method of birth control while you are participating in this study. Also, because the risk is unknown to young children, you should not nurse your baby while in this study. Ask about counseling and more information about preventing pregnancy.
Due to the unknown risks and potential harm to the unborn fetus, sexually active women of childbearing potential must use a reliable method of birth control while participating in this study. Reliable methods of birth control are: abstinence (not having sex), oral contraceptives, intrauterine device (IUD), Depo-Provera, Norplant, tubal ligation, or vasectomy of the partner (with confirmed negative sperm counts) in a monogamous relationship (same partner). An acceptable, although less reliable, method involves the careful use of condoms and spermicidal foam or gel and/or a cervical cap or sponge. We encourage you to discuss this issue further with your physicians if you have any questions.
Pregnant women are excluded from participation in this study. If you are a sexually active woman of childbearing potential and have not been using a reliable method of birth control, two negative pregnancy tests performed 15 days apart are required to check for possible early pregnancy prior to starting treatment.
Risk of Testing for Infectious Illnesses – As part of this study, you will be tested for HIV (human immunodeficiency virus, which is the virus that causes the acquired immunodeficiency syndrome [AIDS]). You will be told of the results of the testing, and counseled as to the meaning of the results, whether they are positive or negative. If the test indicates that you are infected with HIV you will receive additional counseling about the significance of your care and possible risks to other people. We are required by law to report all positive results to the Florida Department of Health. The test results will be kept confidential to the extent permissible under the law. If you do not want to be tested for HIV, you should not agree to participate in this study.
For more information about risks and side effects, ask the study physician or the research coordinator at the time of your review of the Informed Consent Form.
The following tests must be done to make sure that you are eligible for this study. Some of these tests are part of the study and generally would not be part of your routine clinical care. Depending on when you last had them, you may need to repeat some of these tests:
Blood tests
CT/MRI scan of the chest, abdomen, and pelvis
CT/MRI of brain
HIV Test
Pregnancy test if you are of childbearing potential
After screening tests are obtained, it is possible that based on the results you might not be eligible to be treated on this protocol.
Many of these tests will be repeated during the study. If you participate in this study, some of these tests may be done more frequently than if you were not taking part in this research study.
Some of your blood will be sent to a research laboratory to evaluate how well your body has accepted the blood cells from your donor. The blood will be collected prior to beginning treatment on this study, and daily through the date of the last infusion of white cells. In addition, there may be blood draws to check how well white cells in your blood kill cancer cells in the laboratory following white cell transfusions.
You will be asked to consider letting the doctors take a biopsy or fine needle aspiration of your cancer shortly after treatment begins, if it is easily accessible, such as tumors under the skin or in the superficial lymph nodes. If you agree, the biopsy will take place during the first week of your treatment. The researchers in the study will look at the tumor tissue under the microscope to see whether the white cells are surrounding the tumor. You do not have to agree to have the biopsy to participate in this treatment program.
Treatment
Treatment on this research study is an experimental and unproven way to treat cancer. This treatment uses healthy donors’ white blood cells to fight your disease. If you agree to participate, you will receive several infusions of white cells through a line inserted into a vein in your arms, or through the central venous line if available. The number of infusions you will receive will depend on the number of white cells obtained from the donors. You will receive white cell infusion until an adequate number of cells have been infused. We predict that several donors will be required to achieve that cell dose. All of the infusions will be done within a one to two week period.
The white cells transfused are also called granulocytes. They are collected from healthy blood donors using standard granulocyte collection procedures. The donors will be selected and screened by the current standards of blood donations according to the American Association of Blood Banking (AABB) and Food and Drug Administration (FDA). These donors are volunteers, and will be screened by the study team for blood donation eligibility. In addition, these donors will be tested to have compatible blood types (they will be compatible for ABO and Rh blood type with you), to pass a panel of infectious disease blood tests and to have different tissue types or the so-called human leukocyte antigens (HLA) which will lessen the chance of a serious side effect called transfusion-associated graft versus host disease ((TAGVHD )).
Granulocyte transfusions have been used in clinical practice for more than 30 years to treat patients who have life-threatening infections and have very low white cell count following chemotherapy. In that setting, granulocyte infusions are given to help the patients fight infections. In this clinical trial granulocyte infusions are given to hopefully help you fight your cancer.
Donors will be asked to take dexamethasone (a steroid pill) and/or filgrastim (Neupogen) the day before collection of cells. These two medications are widely used in clinical practice to increase the yield of white cells during collection.
Your doctor may give you medications to prevent transfusion reaction, and/or to treat the reaction should it occur. Such medications may include acetaminophen (Tylenol), diphenhydramine (Benadryl) and/or hydrocortisone (a steroid).
You will be monitored closely daily with blood tests during treatment, and monthly after treatment. Your cancer will be assessed three months after the treatment is finished. You might receive other treatments for your cancer if it is getting worse during that time. If your cancer is not getting worse, you will be asked not to take other treatments for your cancer during those three months.
How Long Will I Be in the Study?
We think you will be in the study for approximately 3-5 months. The researcher or your regular doctor may decide to take you off this study if:
The treatment does not work in your cancer.
Your health gets worse.
Your cancer begins to grow.
You can stop participating at any time. However, if you decide to stop participating in the study, we encourage you to talk to the researcher and your regular doctor first.
Request An Appointment
Please fill out the form to request an appointment. We will contact you shortly to confirm the exact day/time.